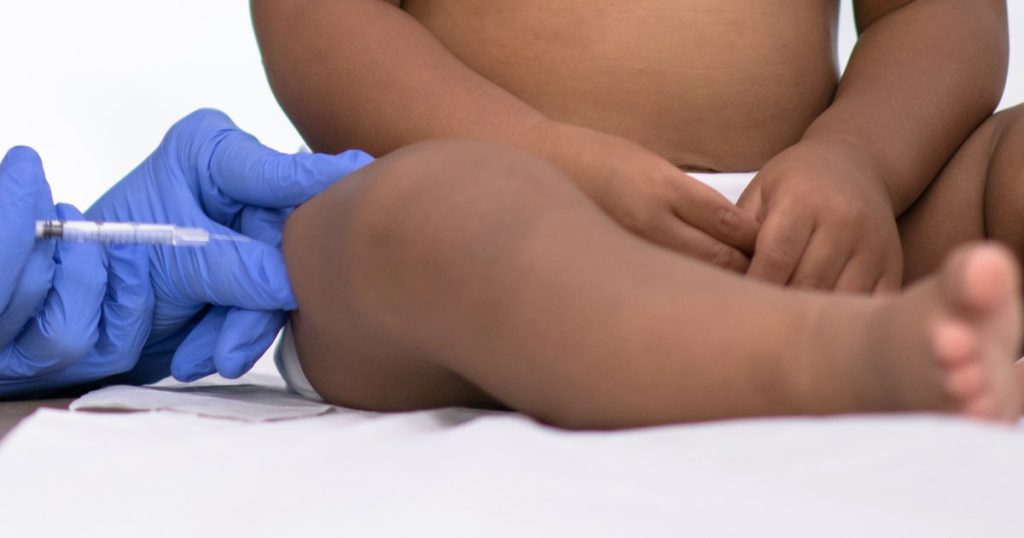
An independent advisory committee to the Food and Drug Administration will decide Thursday whether to recommend an injectable drug that can protect infants up to 2 years old from RSV.
Respiratory syncytial virus causes lower respiratory illness and is most severe for older adults and babies under 6 months old. Among children under 5, the virus leads to up to 80,000 hospitalizations and up to 300 deaths per year in the U.S. Most people are infected by age 2.
The monoclonal antibody drug being considered is administered as a single injection and functions similarly to a vaccine. But instead of prompting the immune system to develop antibodies to the virus, it delivers those antibodies directly to the bloodstream. Scientists refer to this as passive immunization. Monoclonal antibodies were also used to treat Covid before new variants rendered them ineffective.
“It’s a temporary shield, whereas when there’s active immunization you have memory immunity that can be called to the rescue months or years later,” said Dr. Ofer Levy, the director of the Precision Vaccines Program at Boston Children’s Hospital, who isn’t involved in Thursday’s vote.
Active immunization refers to the protection from vaccines, but no RSV vaccine for infants has been approved.
The new monoclonal antibody drug, called nirsevimab, is sold under the name Beyfortus and is already approved in Europe, Canada and the United Kingdom. It was developed by pharmaceutical giant AstraZeneca in partnership with Sanofi.
The injection confers protection to newborns and infants during their first months of exposure to RSV. Children up to age 2 who are vulnerable to severe infections, such as those with chronic heart or lung disease, could get another dose during their second RSV season.
In a study of nearly 1,500 infants, the shot lowered the risk of developing respiratory disease from RSV that required a doctor’s visit by nearly 75% for at least five months.
In a study of more than 1,400 infants born prematurely — a group at particular risk — the injection was found to lower the risk of developing respiratory disease from RSV that required doctors’ visits by around 70% for at least five months. It lowered the risk of hospitalization from RSV by around 78% during that time.
RSV infection rates typically rise in the fall and peak in the winter. However, last year’s season started in June and peaked in November, and resulted in a dramatic spike in severe illnesses that overwhelmed children’s hospitals.
AstraZeneca said Thursday that if its drug gets approved, infants born from April to October could get immunized at their pediatrician’s office before the upcoming RSV season. Infants born from November to March could get immunized after birth, before leaving the hospital.
The company estimated that its injection could prevent up to 500,000 medical visits per year, including up to 60,000 hospital admissions.
In a briefing document, the FDA determined that the injection was safe based on the results of three trials with a total of more than 3,600 participants. Among healthy and preterm infants vaccinated during their first RSV season, a small minority developed a rash as a side effect. Some infants at high risk of severe RSV developed a fever.
The FDA noted that there was a higher number of overall deaths among babies who received the shot compared to those who received a placebo or a different antibody injection, but the agency determined that the discrepancy likely wasn’t related to AstraZeneca’s drug.
The company said Thursday that it should be safe to administer the injection alongside routine childhood vaccines, such as flu shots.
The FDA has already approved one monoclonal antibody injection to protect infants from RSV: a drug called Synagis. But it is only approved for high-risk infants and must be administered monthly during RSV season. The drug lowered the risk of hospitalization from RSV by 45% to 55% in trials.
A vaccine administered to mothers while pregnant in order to protect infants from RSV may also get approved soon: FDA advisers voted last month to recommend that shot. The vaccine, made by Pfizer, lowered the risk of severe disease from RSV among infants by 82% within roughly three months after birth. By around six months, efficacy was around 69%.
The FDA said Thursday that it was unsure whether infants who already had protection from Pfizer’s shot would see an added benefit or new safety concerns if they also got AstraZeneca’s injection.
The FDA approved the world’s first RSV vaccine, for adults ages 60 and up, in early May. Then last week, the agency approved a second vaccine for older adults, which is made by Pfizer and is the same formulation that would be given to pregnant people.